Analyzing Internal Coordinates
Molecular internal coordinates are sets of bond lengths, bond angles and torsion angles that completely define a 3D structure. Internal coordinates see use in a variety of chemical applications and property investigations.
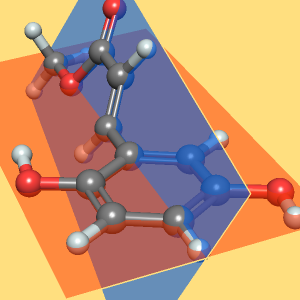
Explore the bond lengths and torsion angles present in the disaccharide maltose.
Use MoleculeValue to find all bond lengths. The list of bond lengths is returned using the efficient QuantityArray structure.
Use MeanAround to find the average bond length.
Use BondList to extract the the atoms and lengths of each bond, then group the bond lengths by member atoms.
Find the average bond length for each bond type.
Finding bond angles is more complex if each angle involves two bonds. First, define a pattern representing any three sequentially bonded atoms.
Use FindMoleculeSubstructure to find the indices of the atoms matching this pattern.
To group the bond angles by atom type, define a sorting function to make sure that CCH and HCC angles are grouped together.
Group the angle positions using the function angleLabel.
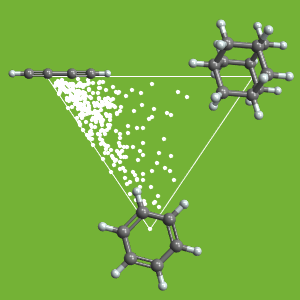
Visualize the variance for different types of bond angles in this molecule.