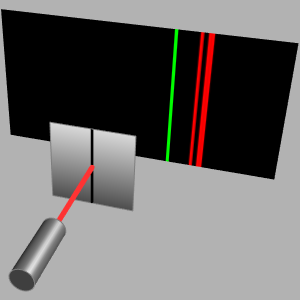
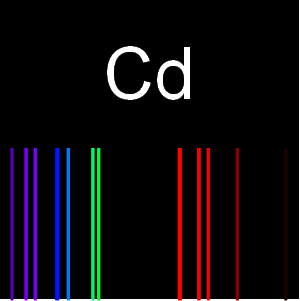
Explore Isotopes and Binding Energies
This example explores the stability and binding energies of various isotopes of known elements. Elements are defined based on the number of protons found in their nucleus. Isotopes of a given element have the same number of protons but varying numbers of neutrons.
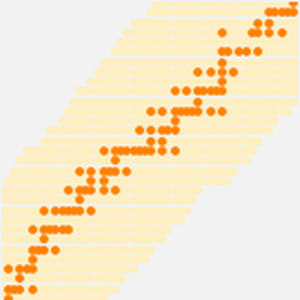
A small fraction of known isotopes are stable. You can observe the variety of isotopes by plotting the number of neutrons versus the number of protons and highlighting the isotopes that are stable.
Stable isotopes are shown in darker orange.
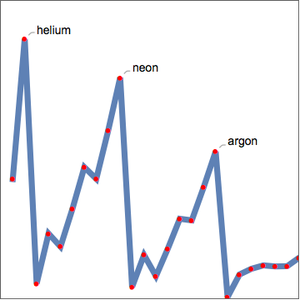
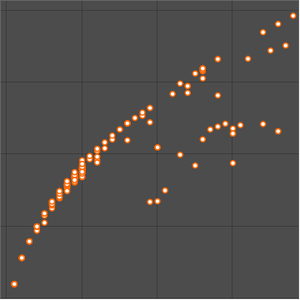
You can also plot the binding energy per nucleon for isotopes.