Experimentally Derive the Periodic Table
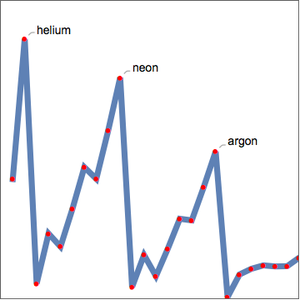
The relationship between elemental property periodicity and the underlying atomic electronic structure can be challenging to visualize. To investigate the relationship between the principle quantum number and ionization energy further, you can examine the first ionization energy for the first 54 elements. If the supposition is correct, the first ionization energies should fall into groups based on the principle quantum number,  , of the outermost electron in each atom.
, of the outermost electron in each atom.
The "IonizationEnergies" property returns a list, so you need to extract just the first ionization energy.
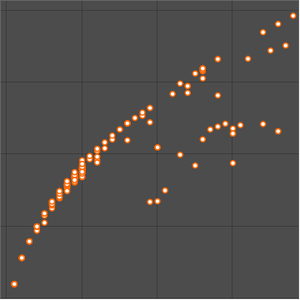
As expected, the first ionization energies fall into five groups corresponding to the principle quantum number. Ionization energy is not the only elemental property exhibiting periodicity. Examine the atomic radius for the same elements.
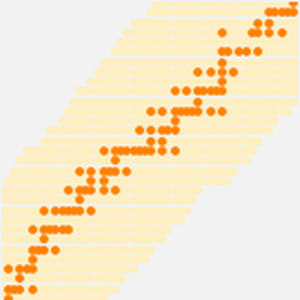
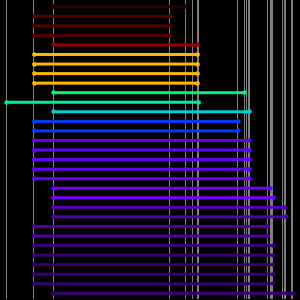
The atomic radius data falls into even cleaner groupings correlating with the principal quantum number of the outermost electron in the atom. These observed principal quantum number groupings suggest an organizational arrangement based solely on observed experimental data. To better visualize this possible organizational arrangement, rotate the atomic radius data, disable data callouts and adjust the aspect ratio of the resulting plot.
What results are five tilted "rows" containing 2, 8, 8, 18 and 18 elements, respectively. Flattening out the rows and squinting just a wee bit, one can see the standard form of the modern periodic table starting to emerge.