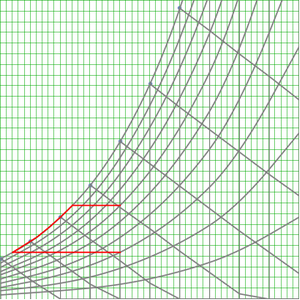
Psychrometric Charts & Dehumidifiers
You can use PsychrometricPropertyData to generate charts about physical properties of gas-vapor mixtures and use those values to determine key properties of psychrometric processes such as dehumidification.
This example calculates the heat flow of an ideal dehumidifier in a room with a temperature of 20 degrees Celsius and a humidity of 60%.
First, calculate the humidity ratio, the ratio between the water content in the air to the mass of the air without that moisture.
Next assume that sensible cooling is used, without altering the moisture content, to reduce the temperature down to the dew point. You can calculate the dew point temperature from the original temperature and relative humidity.
Then the dehumidifier uses latent cooling to reduce the temperature further to 2 degrees Celsius, condensing moisture out of the air. The new humidity ration can be calculated at 2 degrees Celsius and 100% relative humidity.
Thus you have removed half of the water content from the air.
If you were running this dehumidifier in an airtight room without any source of additional moisture, you could calculate the total water collected by the dehumidifier.
This is roughly half a cup.
Returning to the dehumidifier, you now must heat the air back up to 20 degrees Celsius, using sensible heating, to keep the temperature constant. When this is completed, you can calculate the new relative humidity using the new humidity ratio.
You can also estimate heat flow by examining the enthalpy of the condensing and heating process. Calculate the enthalpy at the original temperature and humidity, the final condensation point and the final temperature and humidity.
If you take the difference between the start and end points of the condensation process and multiply by the air mass flow, you can find the heat flow for this process. Assume a standard density for the air and an air flow rate of 1 cubic meter per second.
You can apply the same idea to the heating process, though this time the heat is flowing into the system as opposed to being extracted.