Torsional Potential Energy Surface of Butane
The Wolfram Language features an MMFF94 force field implementation, which is used to minimize the energy for all automatically generated coordinates. To find a conformation with a desired internal coordinate, such as an interatomic distance or angle, it is easy to add a constraint to the built‐in force field.
This example explores the conformational energy of butane by systematically varying the torsion angle around the central carbon-carbon bond, generating a one-dimensional potential energy surface along the way.
Create a molecule for butane.
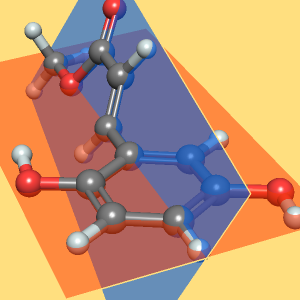
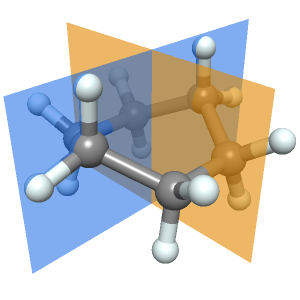
The torsion angle for atoms C1‐C2‐C3‐C4 is the angle between the plane containing atoms C1‐C2‐C3 and the plane containing atoms C2‐C3‐C4. Visualize these planes along with the ball-and-stick model of butane.
Use MoleculeModify to vary the torsion angle, keeping the result for each step in a table.
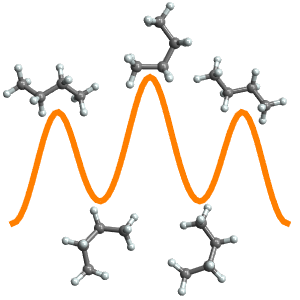
Plot the energy versus torsion angle.
Find the lowest‐ and highest‐energy conformations, typically referred to as "synperiplanar" and "antiperiplanar".